Curriculum
🎯 "Science 10X – Your 5-Step Board Exam Success Plan!"
Chapter 1 : Chemical Reactions and Equations
0/3Chapter 11 : Electricity
0/5Chapter 5 : Life Process
0/1Control and Coordination
0/1Chemical reaction and equation | Most probable questions | Worksheet -1
1. Identify ‘ $p$ ‘, ‘ $q$ ‘ and ‘ $r$ ‘ in the following balanced reaction:
$$p \mathrm{~Pb}\left(\mathrm{NO}_3\right)_{2(s)} \xrightarrow{\text { Heat }} q \mathrm{PbO}_{(s)}+r \mathrm{NO}_{2(g)}+\mathrm{O}_{2(g)} $$
(a) $2,2,4$
(b) $2,4,2$
(c) $2,4,4$
(d) $4,2,2$
[CBSE SQP 2024]
Answer: (a) $2,2,4$
2. Which of the following are combination reactions?
(l) $2 \mathrm{KClO}_3 \xrightarrow{\text { Heat }} 2 \mathrm{KCl}+3 \mathrm{O}_2$
(II) $\mathrm{MgO}+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Mg}(\mathrm{OH})_2$
(III) $4 \mathrm{Al}+3 \mathrm{O}_2 \longrightarrow 2 \mathrm{Al}_2 \mathrm{O}_3$
(IV) $\mathrm{Zn}+\mathrm{FeSO}_4 \longrightarrow \mathrm{ZnSO}_4+\mathrm{Fe}$
Options:
(a) (I) and (III)
(b) (III) and (IV)
(c) (II) and (IV)
(d) (II) and (III)
Answer: (d) (II) and (III)
3. Which among the following statement(s) is/are true? Exposure of silver chloride to sunlight for a long duration turns it grey due to:
(I) the formation of silver by decomposition of silver chloride.
(II) sublimation of silver chloride.
(III) decomposition of chlorine gas from silver chloride.
(IV)oxidation of silver chloride.
Options:
(a) (I) only
(b) (I) and (III)
(c) (II) and (III)
(d) (IV) only
Answer : (a) (I) only
4. In the given experimental setup of electrolysis of water, if P and Q are the gases collected in the test tubes enclosing the electrodes R and S, then select the option/ options in which the matching is correct:
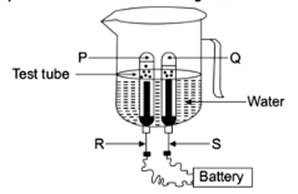
(I) P – Oxygen gas, R – Anode
(II) P – Hydrogen gas,R – Cathode
(III) Q – Hydrogen gas,S – Cathode
(IV) Q – Oxygen gas,S – Anode
Options:
(a) (I) and (II)
(b) (III) and (IV)
(c) (I) and (III)
(d) (II) and (IV)
[CBSE 2025]
Answer: (c) (I) and (III)
5. Assertion (A): Following is a balanced chemical equation for the action of steam on iron.
$$ \begin{aligned} 3 \mathrm{Fe}_{(s)}+4 \mathrm{H}_2 \mathrm{O}_{(g)} \longrightarrow & \mathrm{Fe}_3 \mathrm{O}_{4(s)} + & 4 \mathrm{H}_{2(g)} \end{aligned} $$
Reason (R): The law of conservation of mass holds good for a chemical equation.
Answer these questions by selecting the appropriate option given below:
(a) Both and are true, and is the correct explanation of (A).
(b) Both (A) and (R) are true, and (R) is not the correct explanation of (A).
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true.
Answer: (a) Both and are true, and is the correct explanation of (A).
6. Identify the type of each of the following reactions stating the reason for your answers.
(A) $\mathrm{Fe}_2 \mathrm{O}_3+2 \mathrm{Al} \rightarrow \mathrm{Al}_2 \mathrm{O}_3+2 \mathrm{Fe}+$ Heat
(B) $\mathrm{Pb}\left(\mathrm{NO}_3\right)_2+2 \mathrm{KI} \rightarrow \mathrm{PbI}_2(\downarrow)+2 \mathrm{KNO}_3$
[CBSE SQP 2024]
7. What is the general name of chemicals that are added to fat and oil containing foods to prevent the development of rancidity? [DIKSHA]
8. While Abhi was about to burn magnesium ribbon in the chemistry laboratory, the teacher asked him to clean the ribbon with the sand paper before burning it. What could be the reason for the above instruction by the teacher? After burning the magnesium ribbon, Abhi obtained a white coloured residue. Name this residue. What type of chemical reaction has occurred? Write a balanced chemical equation to explain the reaction. [DIKSHA]
9. (A) Identify the reducing agent in the following reactions:
(i) $4 \mathrm{NH}_3+5 \mathrm{O}_2 \rightarrow 4 \mathrm{NO}+6 \mathrm{H}_2 \mathrm{O}$
(ii) $\mathrm{H}_2 \mathrm{O}+\mathrm{F}_2 \rightarrow \mathrm{HF}+\mathrm{HOF}$
(iii) $\mathrm{Fe}_2 \mathrm{O}_3+3 \mathrm{CO} \rightarrow 2 \mathrm{Fe}+3 \mathrm{CO}_2$
(iv) $2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}$
(B) Define a redox reaction in terms of gain or loss of oxygen.
[CBSE 2023]
10. Write the balanced chemical equations for the given reactions.
(A) Calcium hydroxide + Carbon dioxide $\longrightarrow$ Calcium carbonate + Water
(B) Zinc + Silver nitrate $\longrightarrow$ Zinc nitrate + Silver
(C) Aluminium + Copper chloride $\longrightarrow$ Aluminium chloride + Copper
(D) Barium chloride + Potassium sulphate $\longrightarrow$ Barium sulphate + Potassium chloride